Percent Yield Can Be Described by 1 Word as
The actual yield of a reaction is typically reported as a percent yield or the percentage of the theoretical yield that was actually obtained. What one 1 word can you use to describe the influence of business technologies to the hospitality industry.

How To Calculate Theoretical Yield And Percent Yield Youtube
In chemical reaction engineering yield.

. 113130 x 100 869 2. Revolutionary because it allows the industry to improve its services. My English Language Journey.
A process to produce aluminum from aluminum oxide has an 850 yield. The percent yield is calculated as follows. Assume that the reaction is Al2O3 H2AlH2O 1 See answer ana1147 is waiting for your help.
When the lab actually tried the process out only 134 grams of Miraculosum were collected. These on hydrolysis yield one molecule of a monosaccharose and one of a disaccharose or three of a monosaccharose. Percent Yield The.
But its a flexible formula which means that it doesnt matter which variables you know. 1 grams d If 113 grams of sodium chloride are formed in the reaction described in problem a what is the percent yield of this reaction. Na 2 CO 3 moles0012.
So the calculated mass of the reaction is 1305 g. Add your answer and earn points. The actual mass obtained is 1212 g.
Solve for the theoretical yield of the reaction following all the steps of a stoichiometry. In the following reaction. Na 2 CO 3 g12.
343 Percent yield actual yield theoretical yield 100 percent. Hydrofluoric acid HF are required to react completely with 2368 grams of. CaCl 2 2H 2 O moles0068.
113130 x 100 869 4 Write the equation for the reaction of iron III phosphate with sodium sulfate to make iron III sulfate and sodium phosphate. Write the balanced chemical equation for the reaction. CaCl 2 2H 2 O g68.
Mass of Filter Paper CaCO 3 g. Percent yield experimental mass of the desired product theoretical mass of the desired product 100 For this equation you must know two out of the three valuables. A theoretical yield is calculated by assuming that all the limiting reagent is converted to product.
The percent yield of a reaction is the ratio of the actual yield to the theoretical yield multiplied by 100 to make it a percentage. Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated multiplied by 100. Percent yield is very important in the manufacture of products.
To determine the percent yield and identify how effective the chemical reaction is multiply the decimal percentage of 091244 by 100. 2 FePO 4 3 Na 2 SO 4 Fe 2 SO 4 3 2 Na 3 PO 4. This quiz helps you practice calculating percent error and percent yield in hundreds of chemical reactions.
Much time and money is spent improving the percent yield for chemical production. Heres a quick example. The yield is satisfactory and the wine made the variety known as Gamay noir is described as being like still champagne.
How much aluminum will be produced from a reaction 7000 kg of aluminum oxide to produce Al. 0 In addition trisaccharoses are known of the formula C13H32016. Percent Yield actual yield theoretical yield 100.
Mass of Filter paper g 1g. 0725 moles of aspirin 0725 180 g 1305 g. Calculate the percent yield.
So 0725 moles gives 0725 moles of aspirin. Yield is one of the primary factors that scientists must consider in organic and inorganic chemical synthesis processes. A Word List for Writers.
The percent yield is 134 887 x 100 151. 600 Words to Describe Smiles. Based on this definition we would expect a percent yield to have a value between 0 and 100.
The one word answer can be used in a few ways to provide employee insight. Here are the top 20 words they used to describe their cultures. The percent yield is 912.
The decimal percentage of percent yield is 0 91244. The statement that percent yield can never be greater than theoretical yield is another example of the _____. A ideal gas law B conservation of mass C law of conservation of momentum D law of conservation of energy 1 See answer Advertisement Advertisement.
Identify all important information provided in the word problems or data table. EXAMPLE How many grams of. It also helps the hospitality industry to become more involved in.
The percent yield is the ratio of the actual yield to the theoretical yield expressed as a percentage. Yield actual yieldtheoretical yield 100 So lets say you want to do an experiment in the lab. So the percent yield 1212 1305 100 929.
1291 Percent Yield Actual Yield Theoretical Yield 100. In chemistry yield also referred to as reaction yield is a measure of the quantity of moles of a product formed in relation to the reactant consumed obtained in a chemical reaction usually expressed as a percentage. Words to Describe Your Ideal Employee Therefore my word of intention is ME.
Excess reagent remaining 20 grams 19 grams 130 136 1 grams d If 113 grams of sodium chloride are formed in the reaction described in problem a what is the percent yield of this reaction. The experimentally determined mass of product is then compared to the theoretical yield and expressed as a percentage. A certain chemical reaction was expected to produce 887 grams of the new wonder drug Miraculosum.
Of the actual yield to the theoretical yield expressed as a percentage. The influence of business technologies on the hospitality industry can be described in one word.
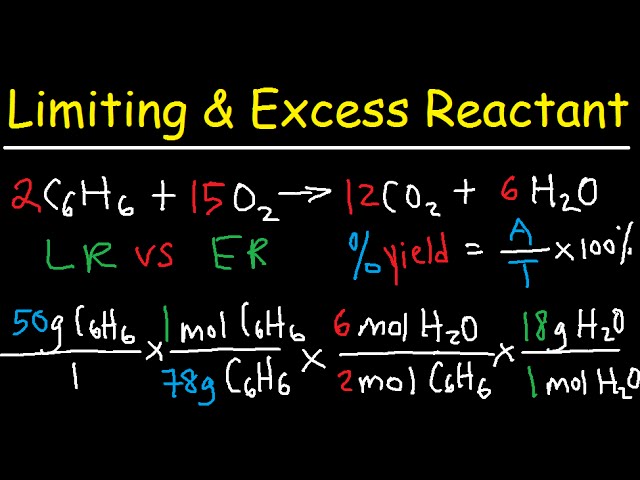
Stoichiometry Limiting Excess Reactant Theoretical Percent Yield Chemistry Youtube

Notes On Percent Yield And Mass To Mass Stoichiometry With Examples And Pract Scientific Notation Word Problems Persuasive Writing Prompts Chemistry Worksheets

Percent Yield Percent Purity Video Lessons Examples And Solutions
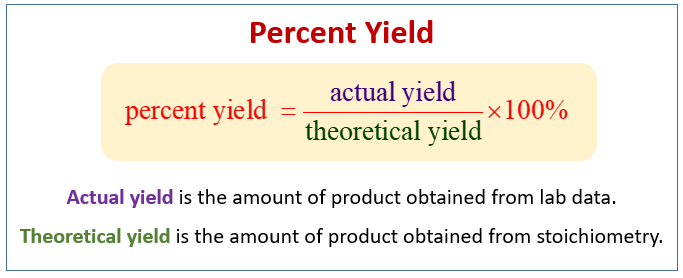
Stoichiometry And Percent Yield Examples Solutions Worksheets Videos Games Activities

Percent Yield Tutorial Explained Practice Problems Crash Chemistry Academy Youtube
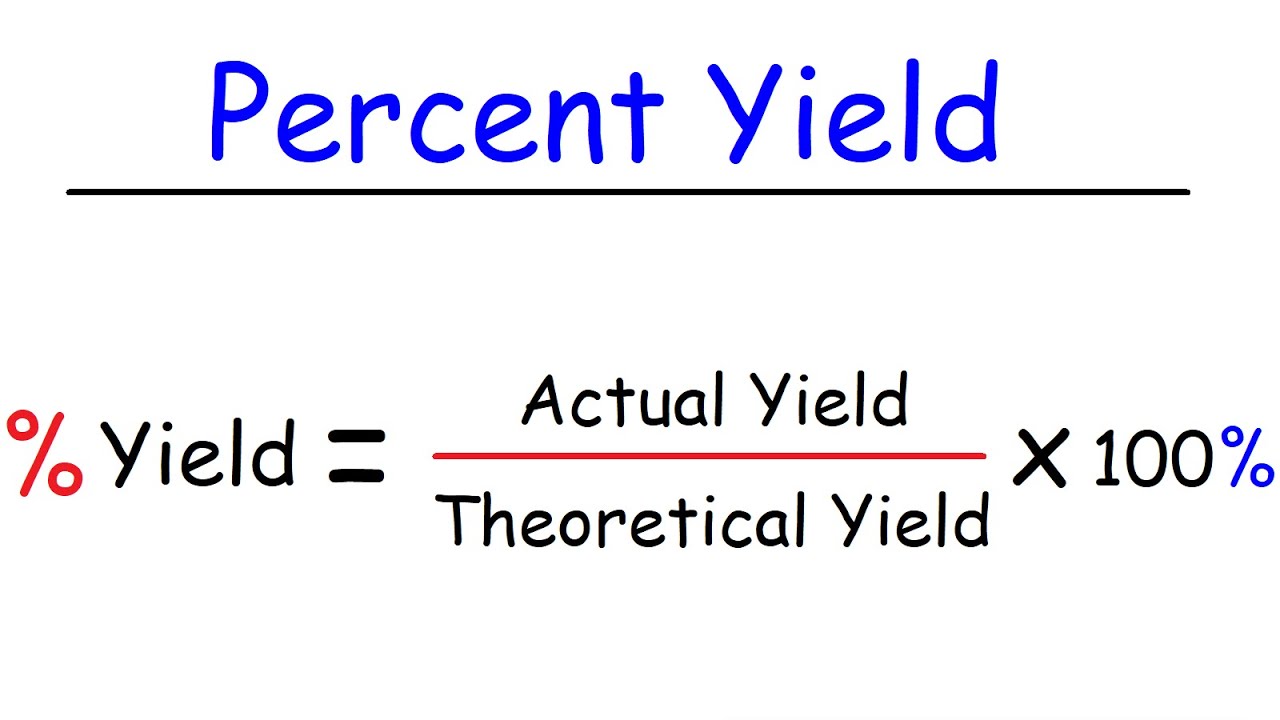
How To Calculate Theoretical Yield And Percent Yield Youtube

Stoichiometry Percent Yield Practice 2 Al 6 Hcl 2 Alcl H 2 95 G Of Hydrochloric Acid Are Reacted With Excess Aluminum Producing 2 2 G Of Hydrogen Ppt Download

Stoichiometry Worksheet Answer Key Fresh Stoichiometry Worksheet 2 Percent Yiel Persuasive Writing Prompts Scientific Notation Word Problems Persuasive Writing

Calculating Percent Yield Chemtalk

Formal Lab Report Template Lovely Bacteriology Lab Report Lab Report Template Report Template Lab Report
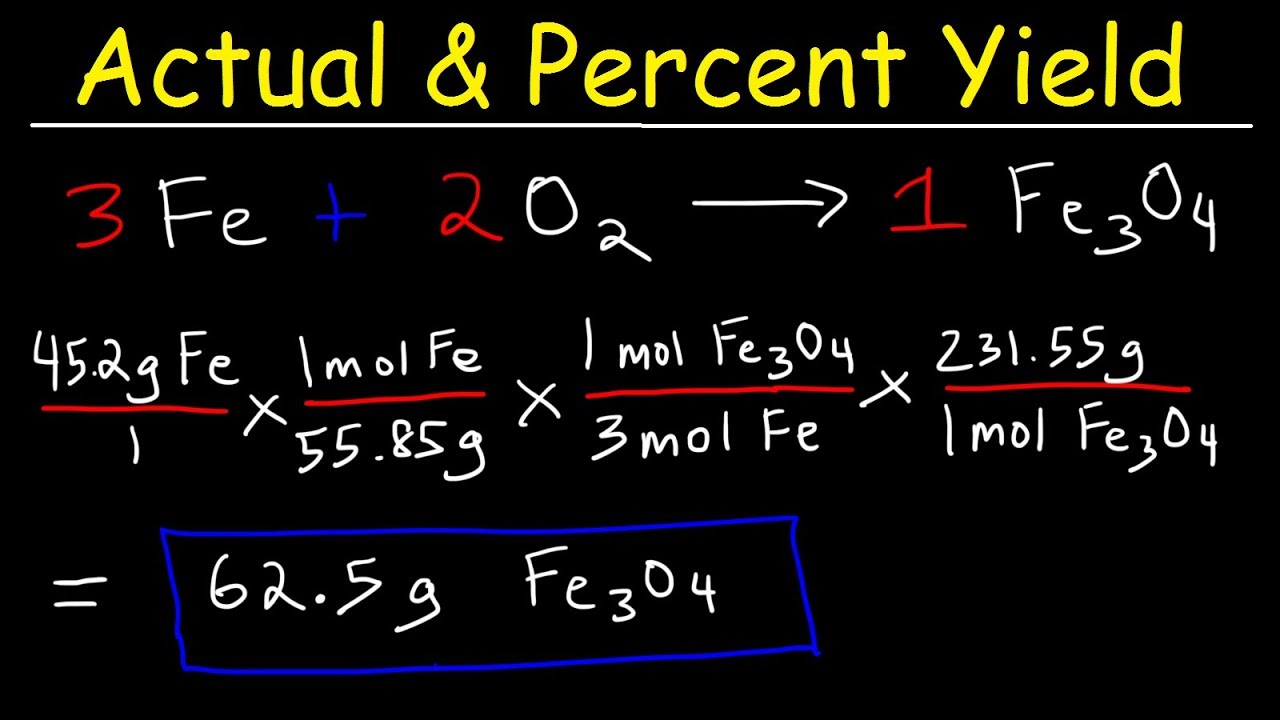
How To Calculate The Percent Yield And Theoretical Yield Youtube

How To Find Actual Yield Theoretical Yield And Percent Yield Examples Practice Problems Youtube

Stoichiometry Percent Yield Practice 2 Al 6 Hcl 2 Alcl H 2 95 G Of Hydrochloric Acid Are Reacted With Excess Aluminum Producing 2 2 G Of Hydrogen Ppt Download

Stoichiometry Percent Yield Practice 2 Al 6 Hcl 2 Alcl H 2 95 G Of Hydrochloric Acid Are Reacted With Excess Aluminum Producing 2 2 G Of Hydrogen Ppt Download
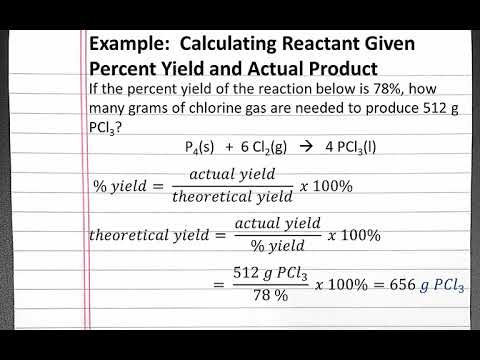
Chemistry 101 Calculating Reactant Given Percent Yield And Actual Product Youtube

Theoretical Actual And Percent Yield Problems Chemistry Tutorial Youtube

Chemistry 101 Calculating Reactant Given Percent Yield And Actual Product Youtube

Stoichiometry Percent Yield Practice 2 Al 6 Hcl 2 Alcl H 2 95 G Of Hydrochloric Acid Are Reacted With Excess Aluminum Producing 2 2 G Of Hydrogen Ppt Download

Gotempl Statement Template International Bank Bank Statement
Comments
Post a Comment